by admin | Aug 7, 2015 | Uncategorized
On July 13, 2011, the Food and Drug Administration (FDA) issued an urgent public notice advising patients and their healthcare providers to consider alternatives to transvaginal mesh. The advisory also noted that the FDA will be meeting to discuss a potential ban on...
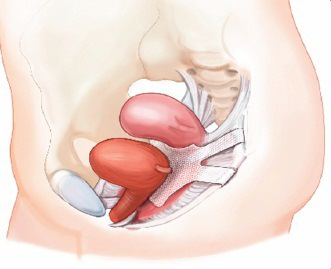
by admin | Aug 7, 2015 | Uncategorized
On July 13, 2011 the FDA released a Safety Communication regarding the usage of synthetic mesh materials for the repair of pelvic organ prolapse. While the FDA has neither taken the vaginal mesh off the market nor called for a recall, they did state that a variety of...
by admin | Aug 7, 2015 | Uncategorized
On August 25, 2011 Public Citizen, a consumer advocacy group representing more than 225,000 members and supporters nationwide, petitioned the Food and Drug Administration (FDA), pursuant to the Medical Device Amendments to the Federal Food, Drug, and Cosmetic Act, 21...
by admin | Aug 7, 2015 | Uncategorized
On January 20, 2012, Representative Henry Waxman of California led House Democrats from the Energy and Commerce Committee in writing a letter to several chairmen, requesting that the Committee hold hearings to review the safety of medical devices implanted into...
by admin | Aug 7, 2015 | Uncategorized
A nationwide class action settlement with an estimated value of over $40 million was approved on April 13, 2012 by United States District Judge Katherine S. Hayden of the District of New Jersey, in the case of Alin v. American Honda Motor Co., Inc., Civ. No....



Recent Comments